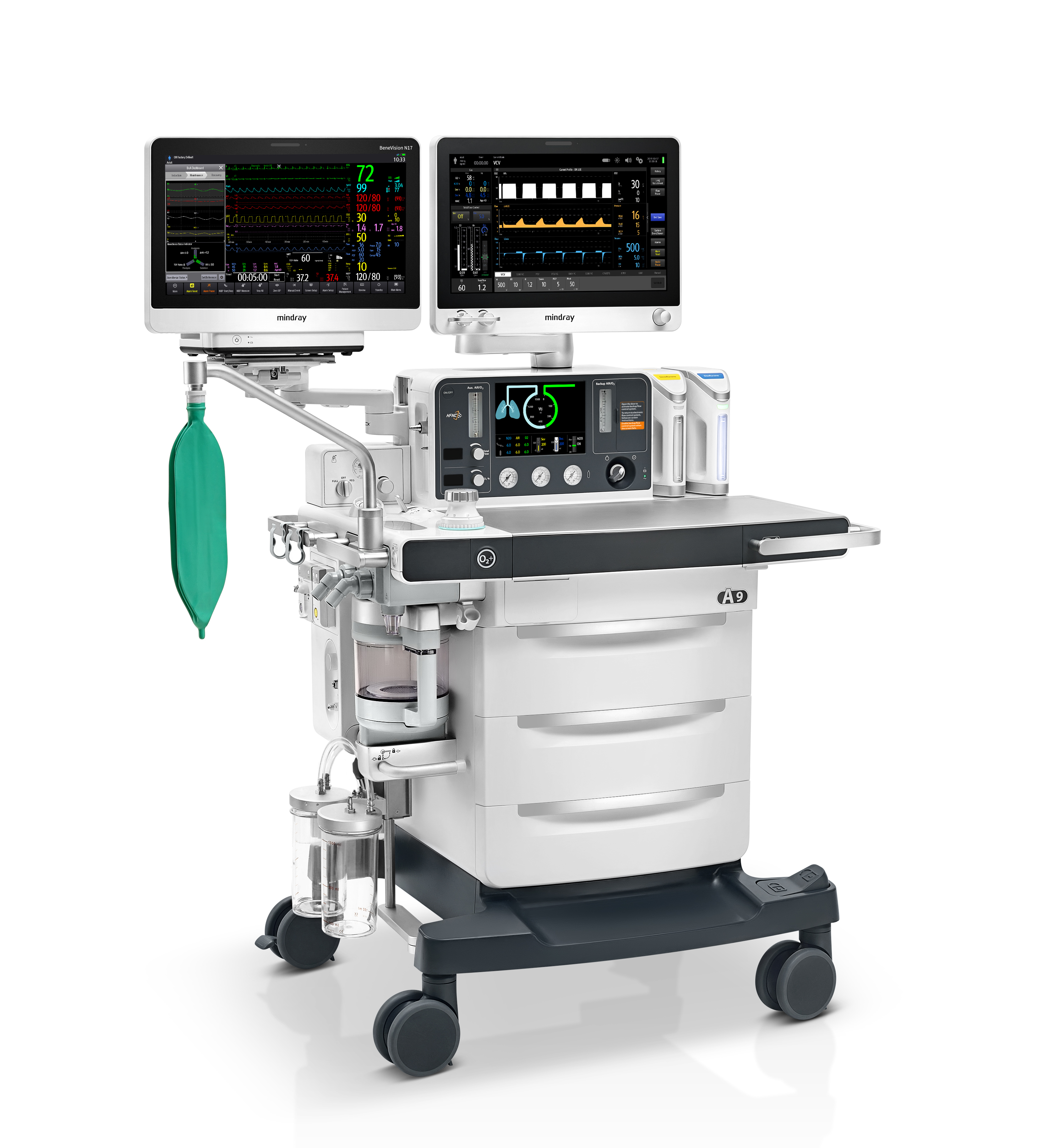












A9 Anaesthesia System
Medical device
Shenzhen Mindray Bio-Medical Electronics Co., Ltd.Patient safety is the top priority in the operating room while human error is one of the biggest threats. The anesthesia system is a vital device used by anesthesiologists to protect patient safety. Mindray has developed a product combining classic and high-tech. The A9 not only applies cutting-edge technologies for intelligent anesthesia but also retains traditional functions to suit clinicians’ habits. Its unique structures make it 360-degree rotatable for different clinical scenarios, and the flexible integrated solutions of other devices can meet various clinical requirements. These designs minimize human error to guarantee patient safety during surgery.
Client / Manufacturer Design
Design

Shenzhen Mindray Bio-Medical Electronics Co., Ltd.
Shenzhen, CN
Shenzhen Mindray Bio-Medical Electronics Co., Ltd.
Shenzhen, CNDate of Launch
2020
Development Time
25 - 36 months
Target Regions
Africa, Asia, Europe, North America, South America
Target Groups
Consumer / User, Trade / Industry, Other target groups: Medical staffs