
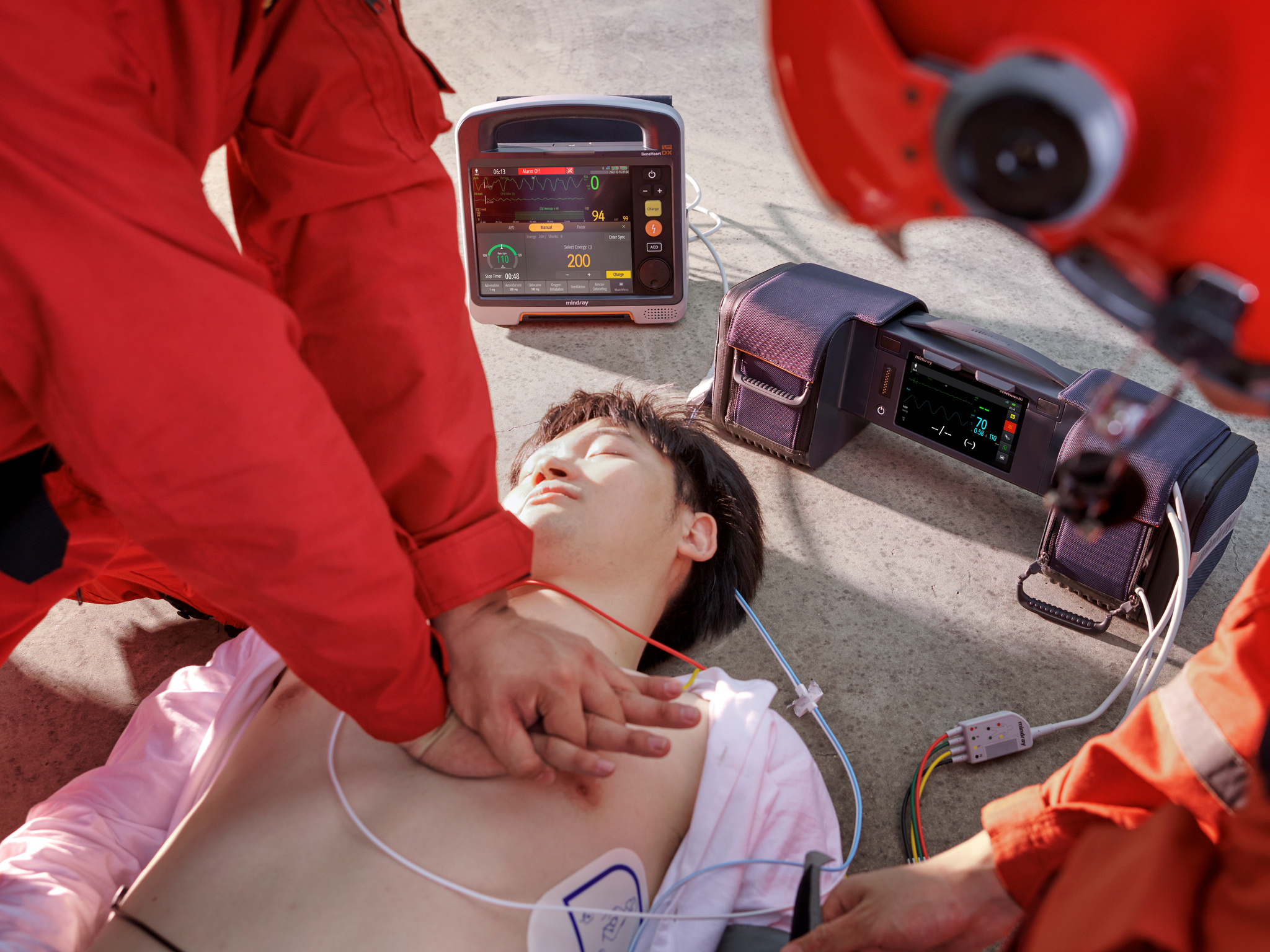








BeneHeart D Series
Defibrillator Monitor
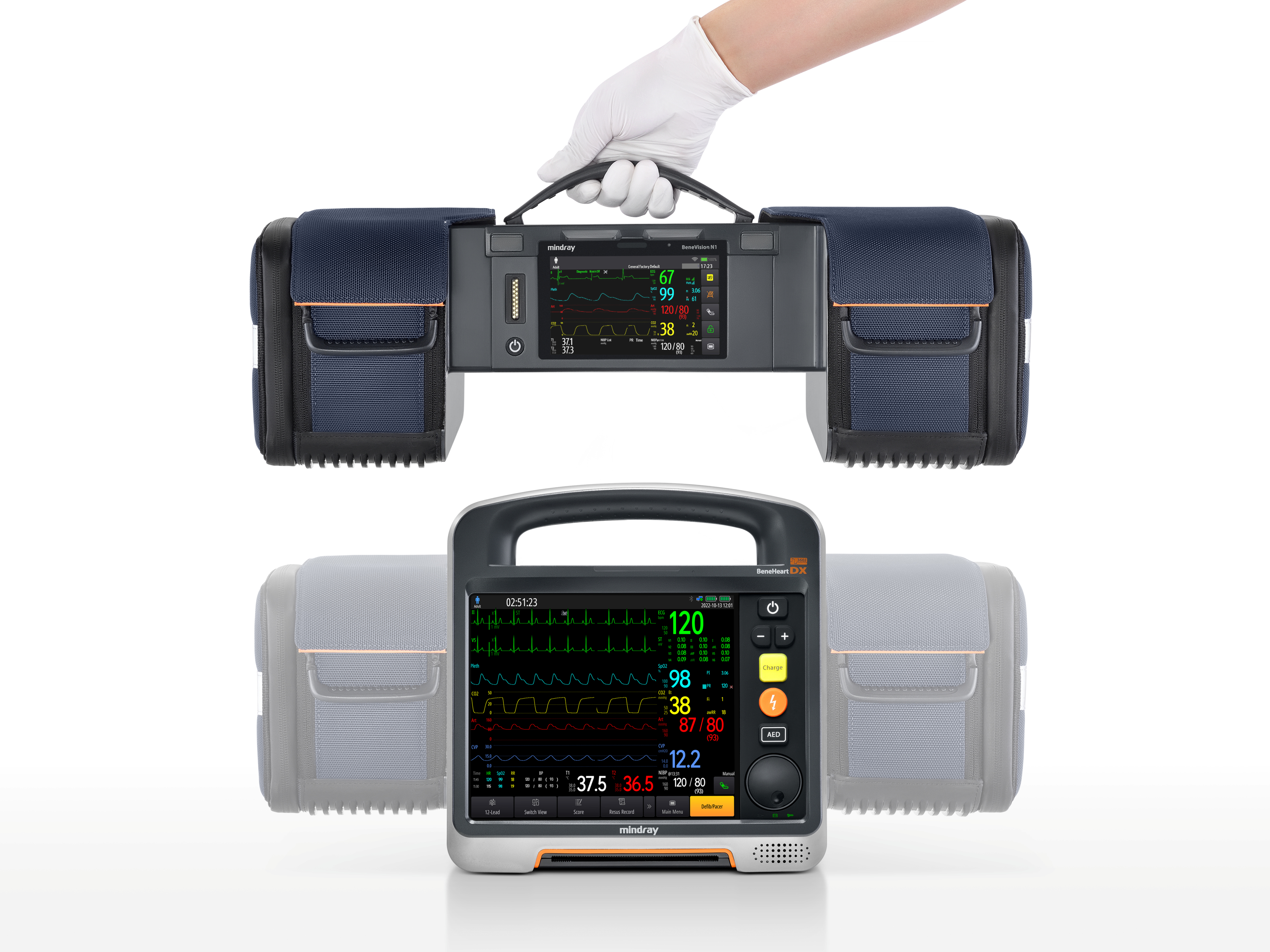
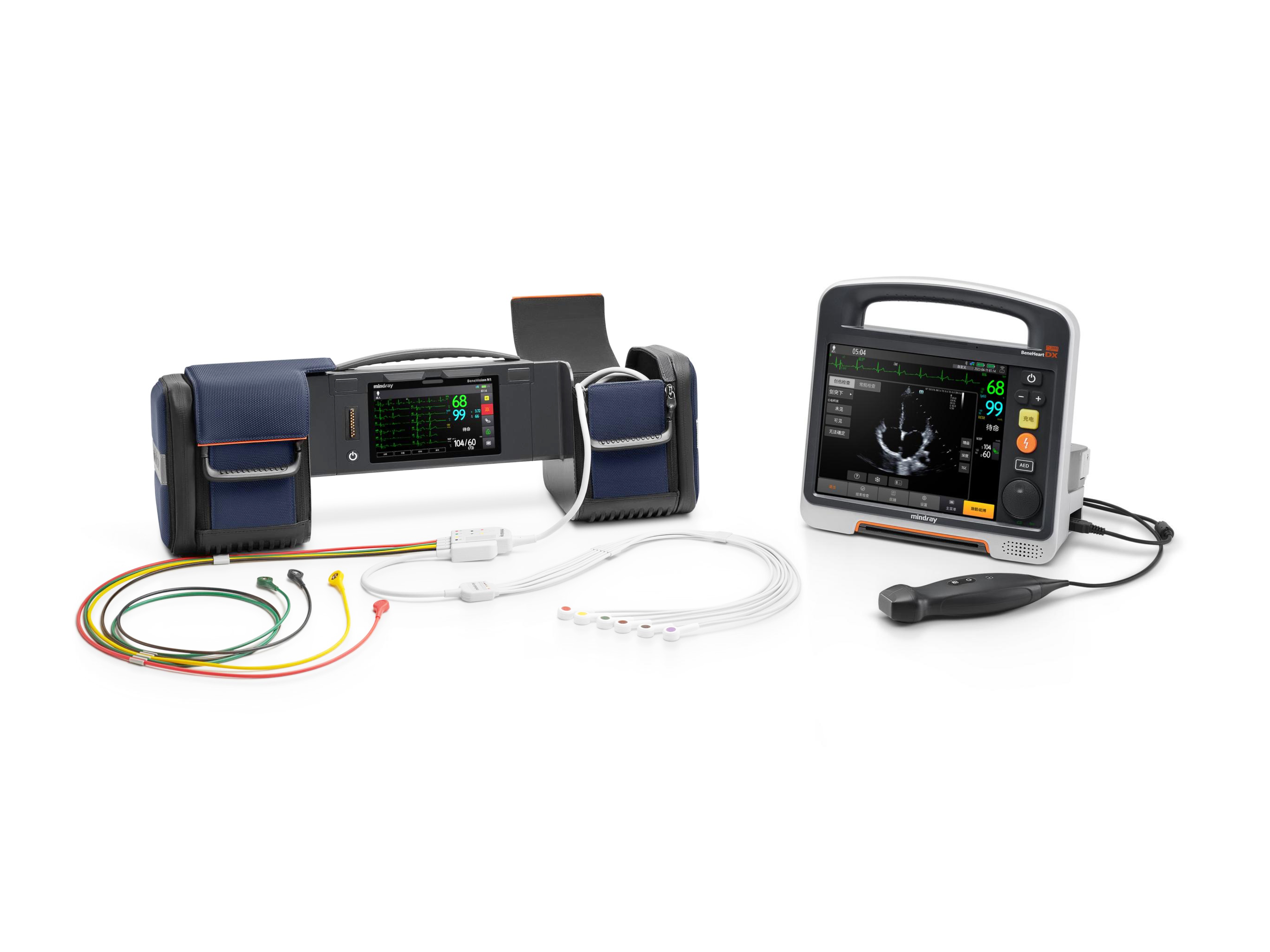
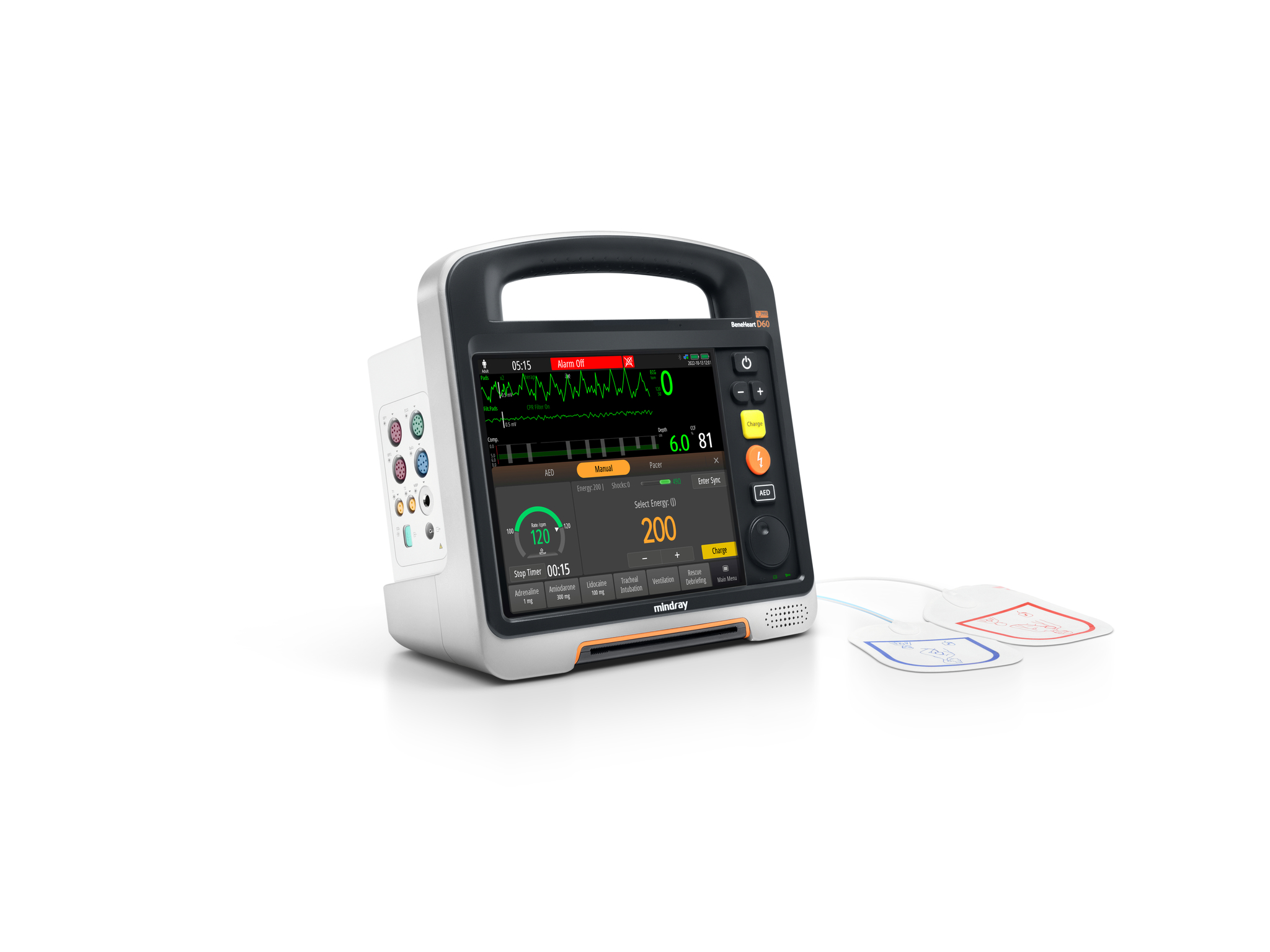
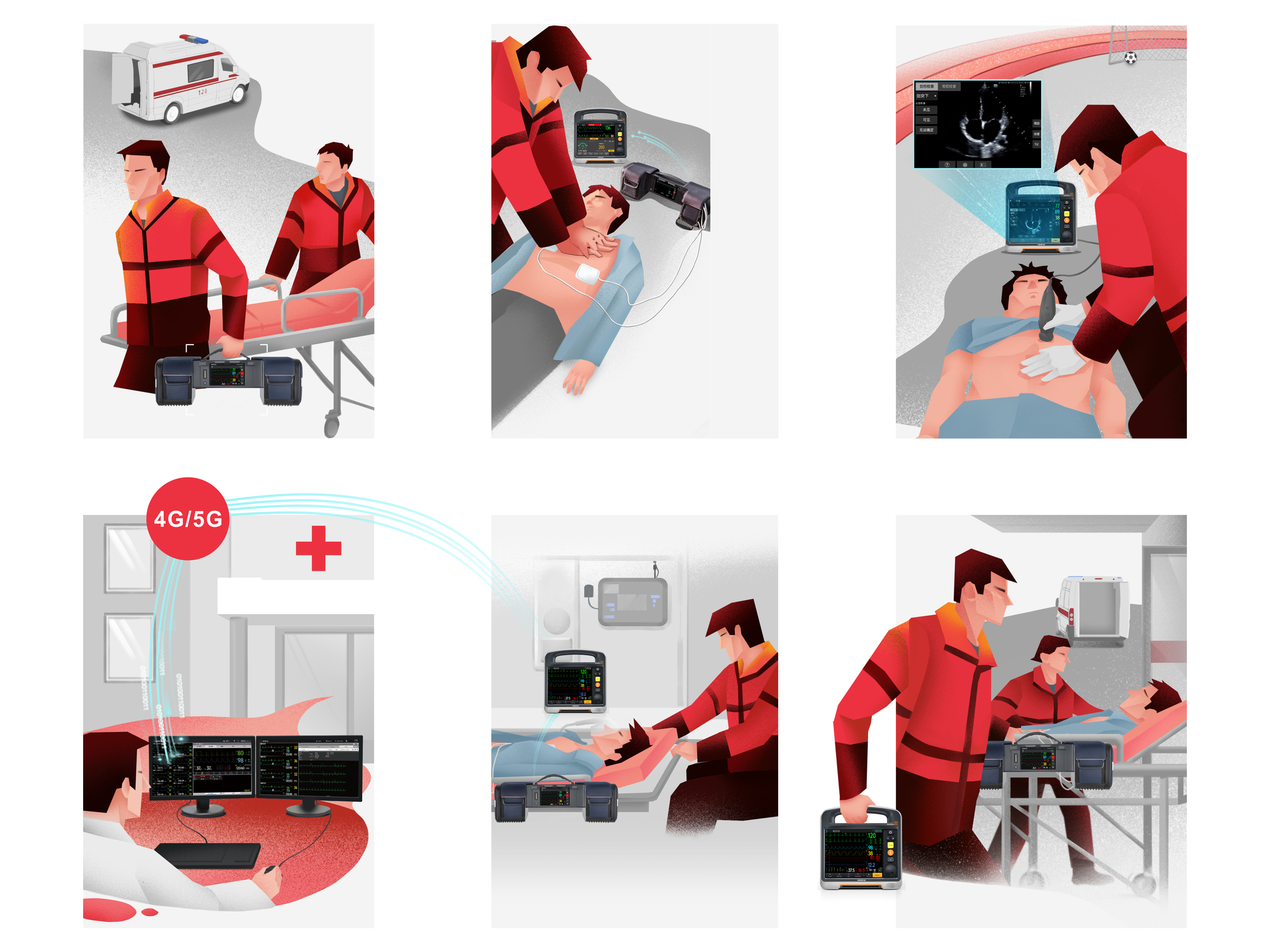
Shenzhen Mindray Biomedical Electronics Co., Ltd.BeneHeart D Series defibrillator monitor has the world's leading 'modular clinical toolkit’ concept, which can adapt to various and complex emergency scenarios. The innovative modular design allows flexibly split and combine device to adapt to different rescue and patients, solves the problem of emergency device being large and heavy, diverse, and difficulty in carrying. At the same time, the unique information network system and the transport monitor N1 in the module have improved the efficiency in receiving patients and the integrity of data, making pre and in-hospital rescue connections safer and more efficient.
Client / Manufacturer Design
Design

Shenzhen Mindray Biomedical Electronics Co., Ltd.
Shenzhen, CN
Shenzhen Mindray Biomedical Electronics Co., Ltd.
Shenzhen, CNHuan Zou, Lijuan He, Lei Zhang, Xiaoling Zou, Zining ChenDate of Launch
2023
Development Time
more than 24 Month
Target Regions
Africa, Asia, Australia / Oceania, Europe, North America, South America
Target Groups
Consumers / Users, Trade / Industry, Public Sector / Government