











dermaject
Injection device
Hahn-Schickard
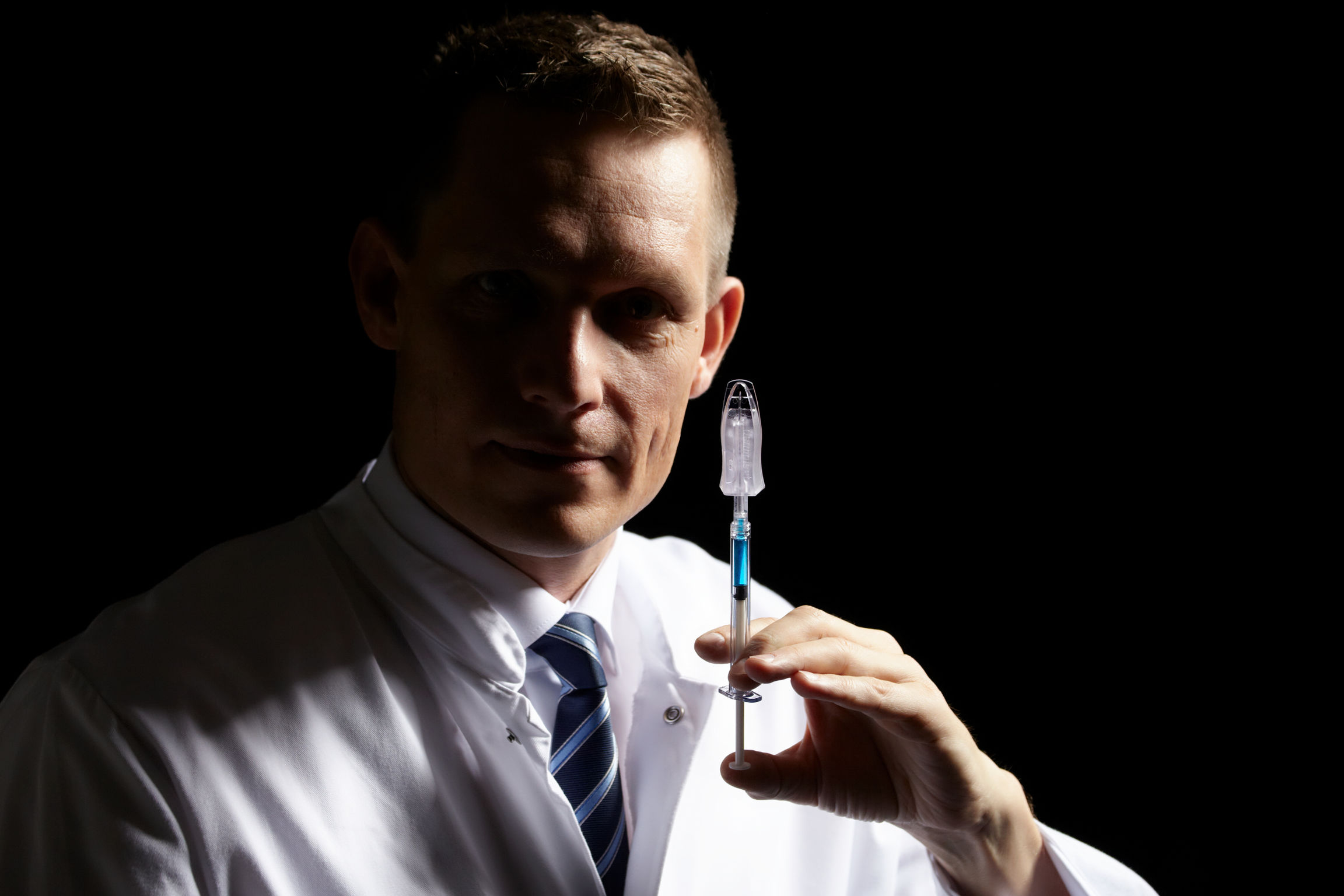
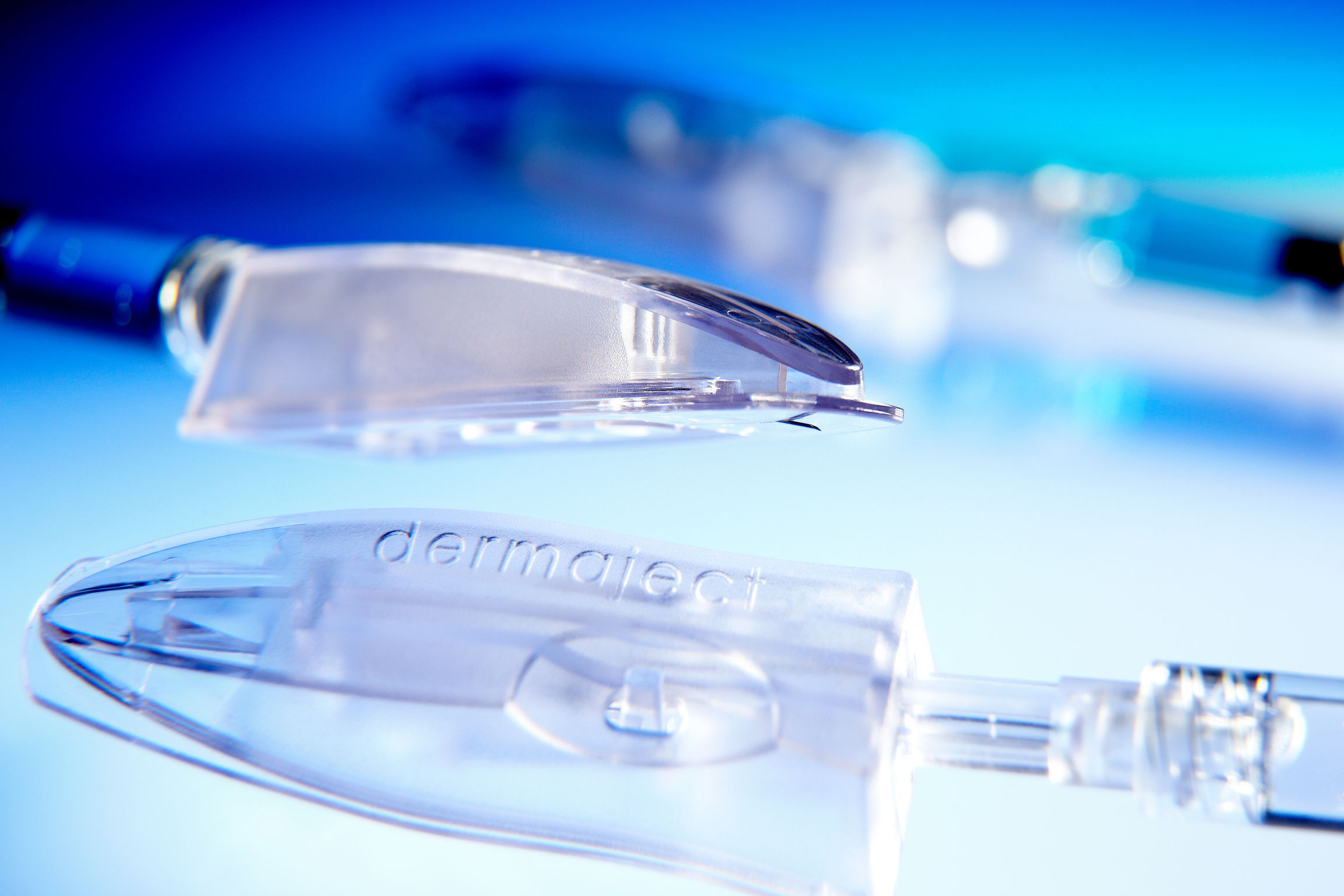
Dermaject is a specialty drug delivery device for the injection of liquid drugs, such as therapeutic cancer treatments into the upper layer of the skin (the dermis, just below the skin surface). Most intradermal (i.d.) injections are carried out manually with painful thick needles and an error rate of about 20%. Dermaject standardizes injections, reduces errors, is 2 times faster and, thanks to a needlestick injury shield, is far less painful. Intradermal injections using dermaject exhibit up to 30x increased efficacy compared to the normal subcutaneous route. It is also the only device mimicking the Mantoux method of sharp angle entry.
Client / Manufacturer Design
Design
Hahn-Schickard
Villingen-Schwenningen, DE
Verapido Medical GmbH
Villingen-Schwenningen, DE
Verapido Medical GmbH
Villingen-Schwenningen, DEDate of Launch
2017
Development Time
25 - 36 months
Target Regions
Asia, Australia/Oceania, Europe, North America
Target Groups
Trade / Industry, Specific sub-group: Pharma