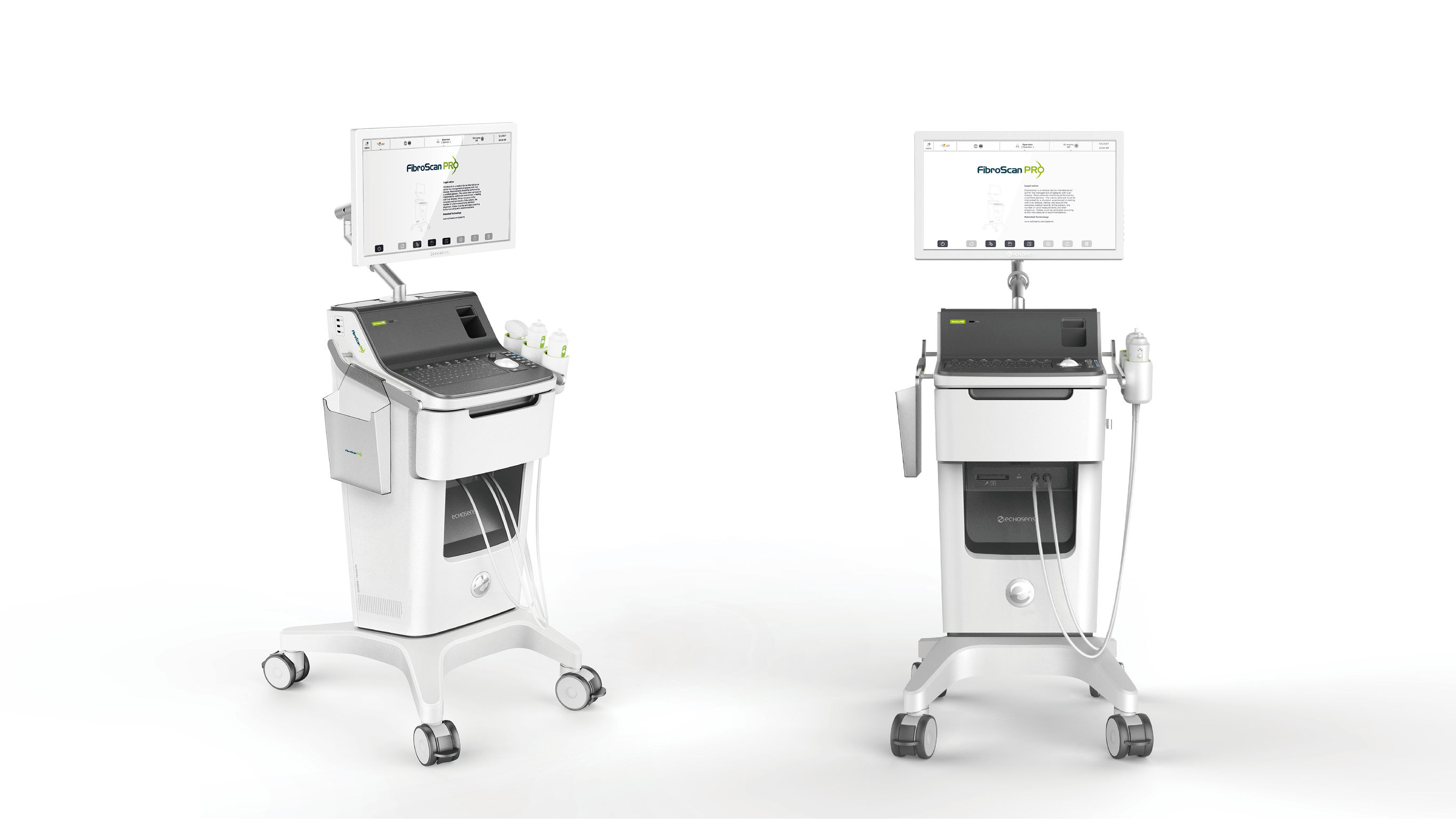



FibroScan PRO
Medical device
Echosens Shenzhen Healthcare Co., Ltd.
FibroScan PRO is a unique medical device used in research and clinical practice to assess liver health status in patients with chronic liver diseases. It measures liver fibrosis and liver steatosis. The method is non-invasive and painless. It requires only a few minutes and is very well accepted by patients. It is based on a disruptive and patented technology: Vibration-Controlled Transient Elastography (VCTE). Its front drawer can store probes, coupling agents and wipes in a safer and more hygienic way; the screen can be adjusted flexibly and the control panel offers related shortcuts, resulting in significantly improved work efficiency.
Client / ManufacturerDesign
Echosens Shenzhen Healthcare Co., Ltd.
Shenzhen, CNND Industrial Design Co., Ltd.
Shenzhen, CNDate of Launch
2018
Development Time
25 - 36 months
Target Regions
Asia
Target Groups
Other target groups: doctors & patients, Specific sub-group: doctors & patients