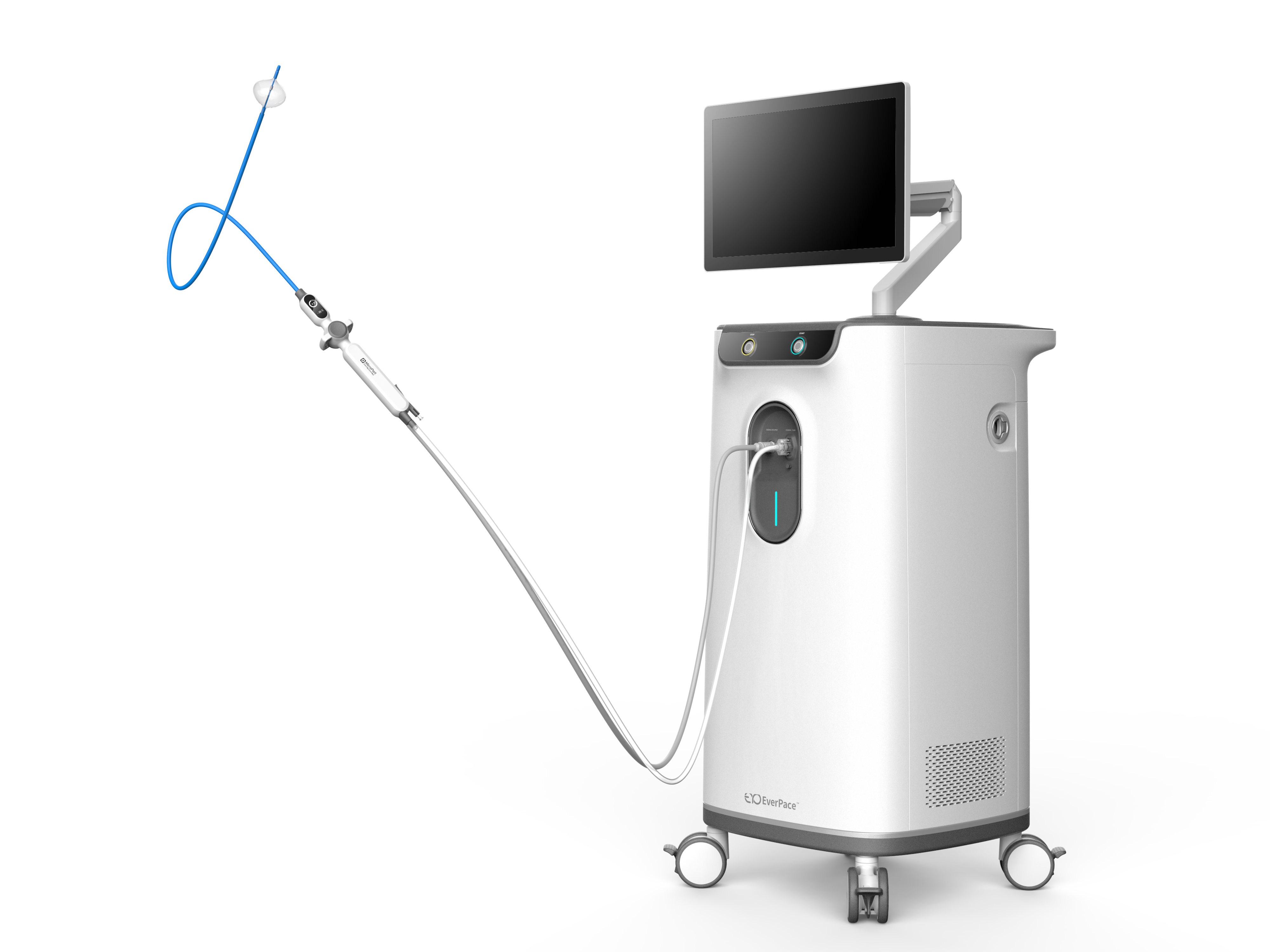



IceMagic CryoAblation System
Medical Equipment
Shanghai MicroPort EP Medtech Co., Ltd.
Atrial fibrillation is a progressive disease, meaning that patients experience more frequent and longer episodes over time, and medications as well as catheter ablation may become less effective. Only after experiencing AAD failure do some patients proceed with catheter ablation. Coupled with growing clinical evidence, proves that cryoablation as a first-line treatment is a more effective solution for preventing recurrent atrial arrhythmias. Effectively shorten the operation time, better treatment effect, less postoperative complications.
Client / ManufacturerDesign
Shanghai MicroPort EP Medtech Co., Ltd.
Shanghai, CN
MicroPort Sinica Co., Ltd.
Shanghai, CNMicroPort Design CenterDate of Launch
2023
Development Time
up to 36 Month
Target Regions
Asia, Europe
Target Groups
"Medical"