





OMRON WheezeScan HWZ-1000T
Child's asthma management system
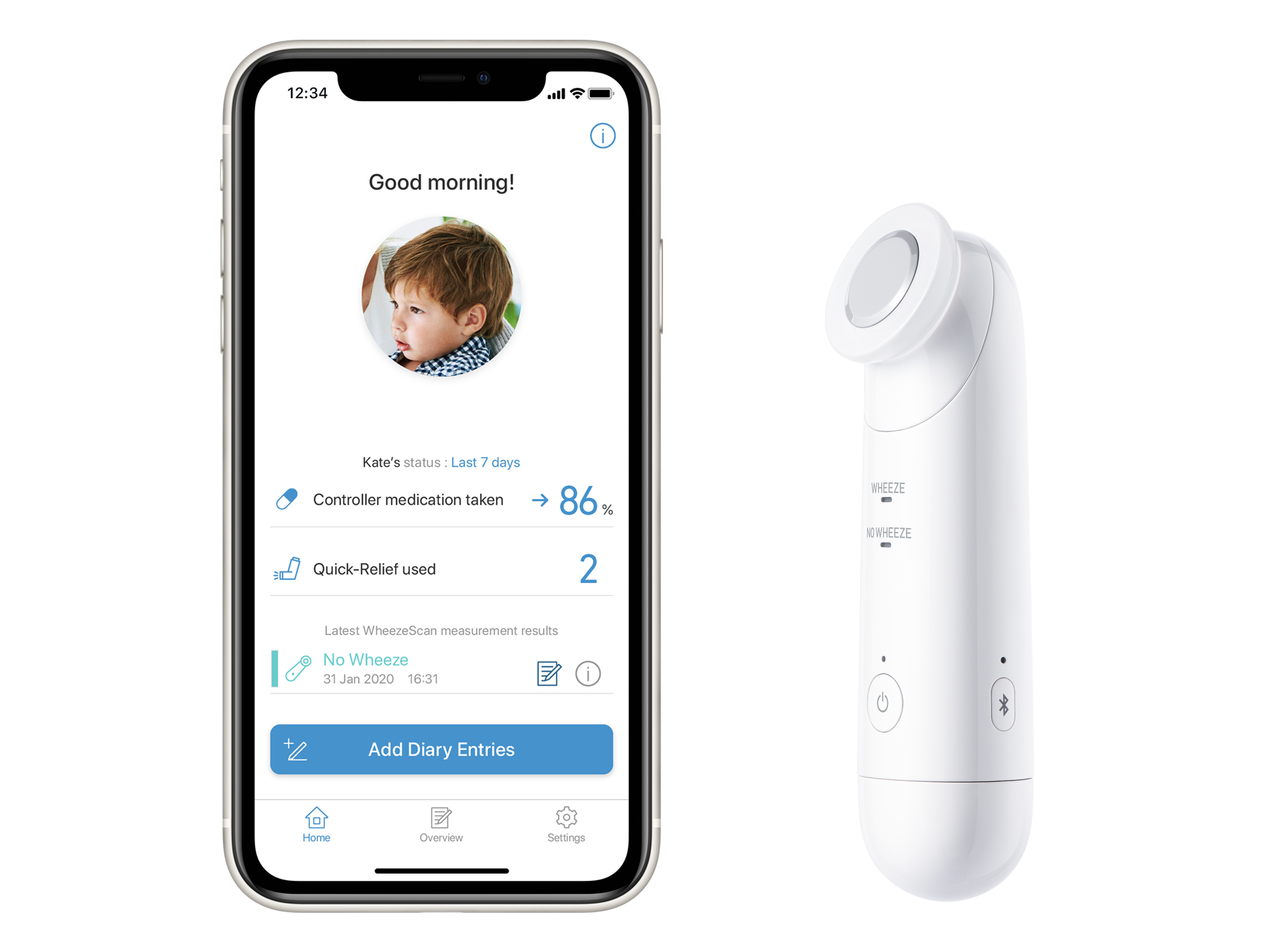
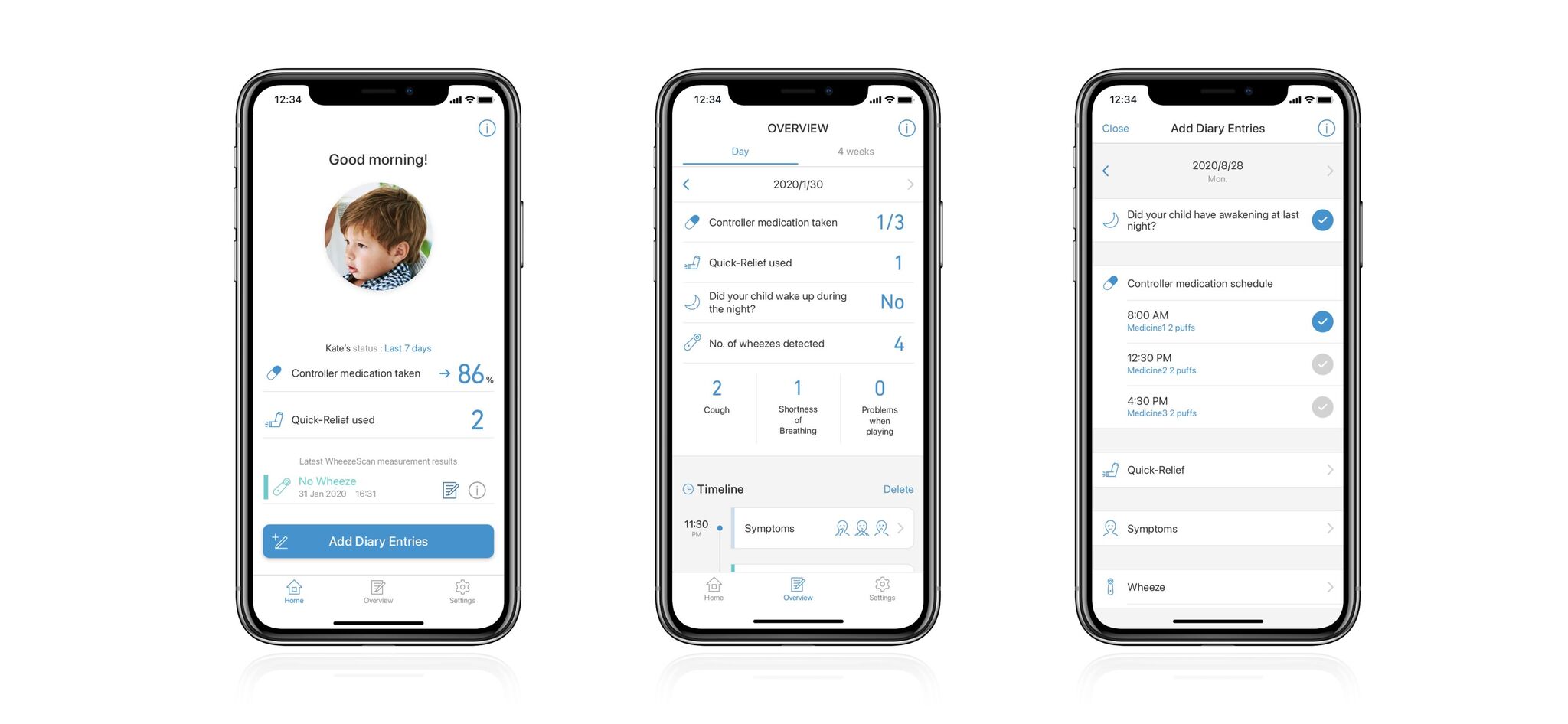
OMRON HEALTHCARE Co., LtdThis new child asthma management system uses WheezeScan to detect wheezing (abnormal breathing sounds) in children. Because children cannot accurately communicate their asthma symptoms, it can be difficult to properly administer medication before symptoms worsen. WheezeScan checks for wheezing, enabling proper treatment before the child's asthma becomes acute. The Asthma Diary application records daily symptoms and prescribed medication. When used together to record wheezing occurrences, symptoms, and medications, the system accurately shares home asthma management information with a doctor.
Date of Launch
2020
Development Time
25 - 36 months
Target Regions
Asia, Europe, North America
Target Groups
Consumer / User, Other target groups: Hospitals, corporations and Corporations, insurance companies
