
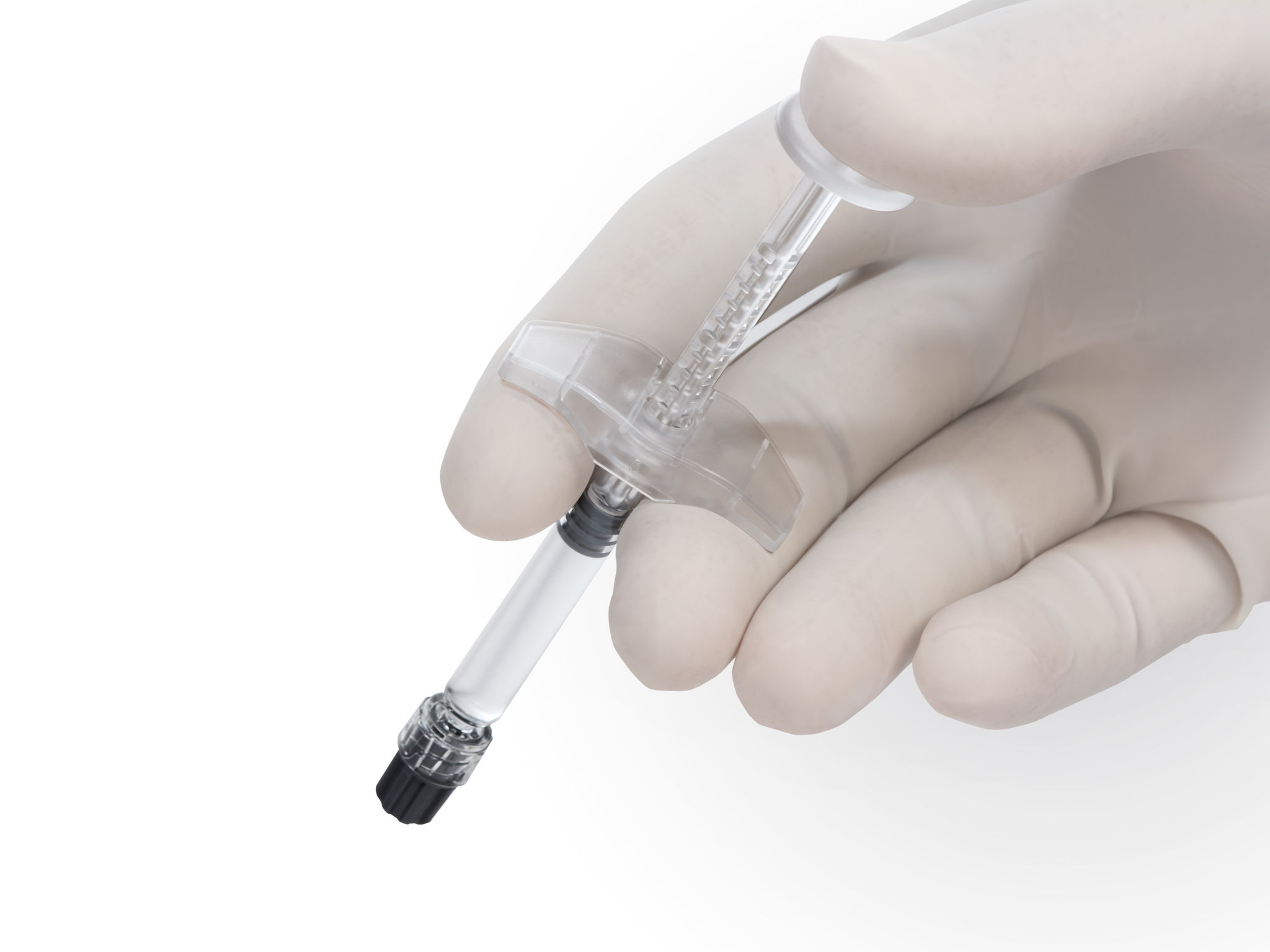


Quatroject
Piston and backstop
Croma-Pharma GmbH
The new intradermal syringe features a unique design, which is the basis for a high degree of recognition in the field of esthetic medicine. The design of the backstop and the piston rod was developed and tested together with dermatologists and plastic surgeons to enable optimal and safe handling. Furthermore, the components can now be used in a fully automated production process, which increases safety and quality of the final products significantly. The Polycarbonate material is of medical grade (ISO 10993 and USP Plastics Class 6 conform) and meets the high requirements for medical devices.
Client / ManufacturerDesign
Croma-Pharma GmbH
Leobendorf, ATCroma-Pharma GmbH
Leobendorf, ATDate of Launch
2017
Development Time
13 - 24 months
Target Regions
Africa, Asia, Australia/Oceania, Europe, North America, South America
Target Groups
"doctors from the dermatology and aesthetic medicine"