










Resona A20
Ultrasound Diagnostic System
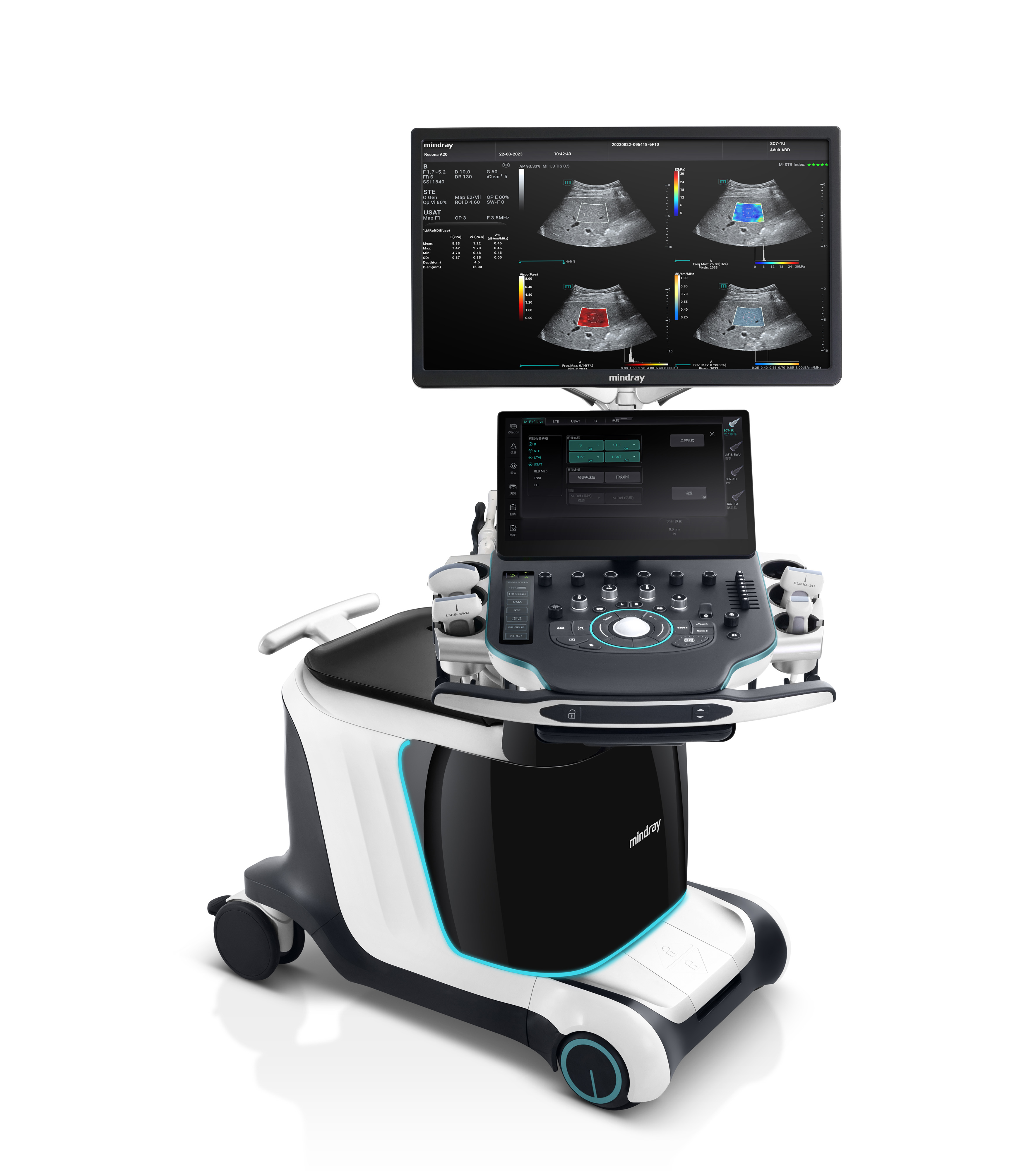
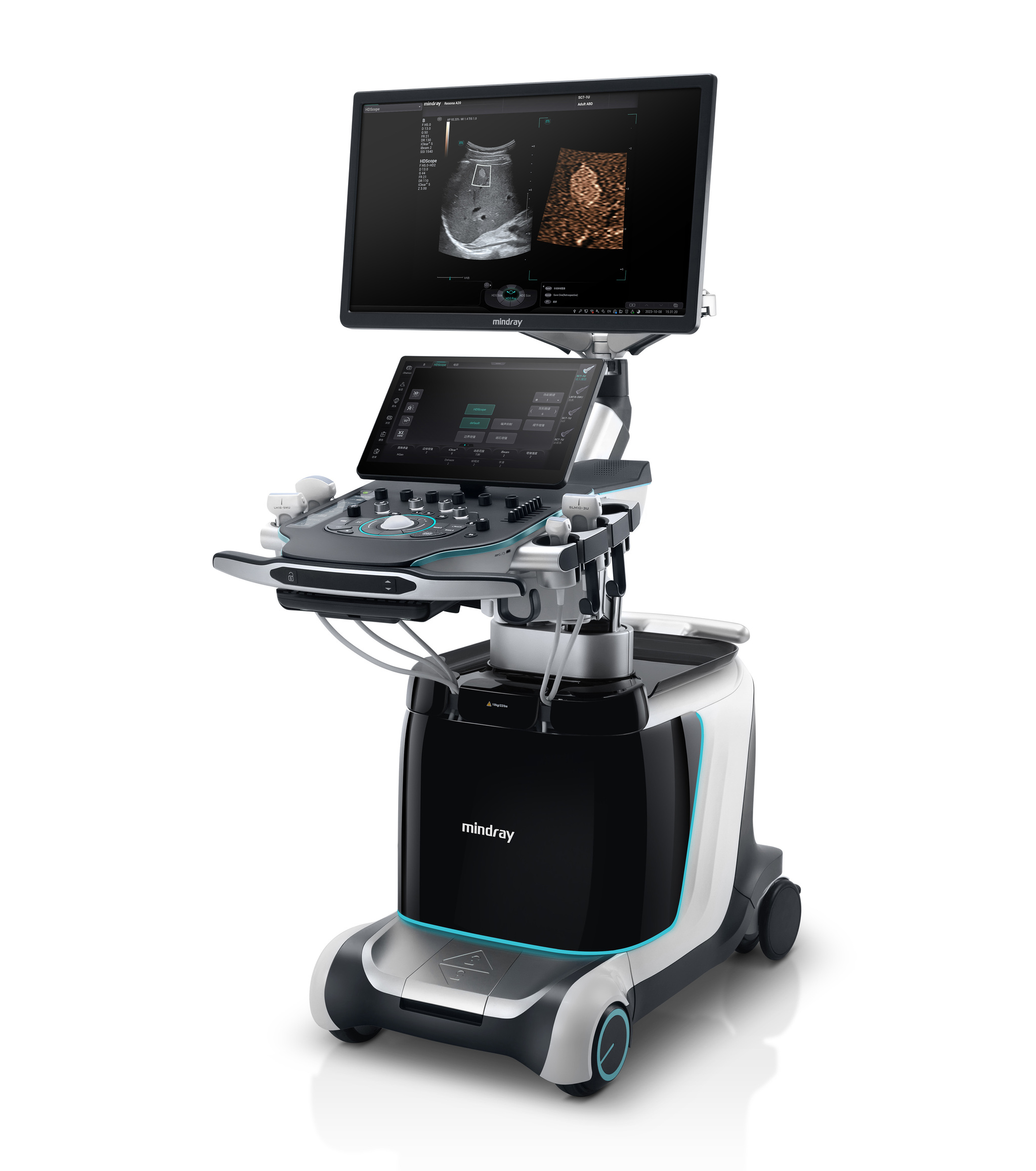
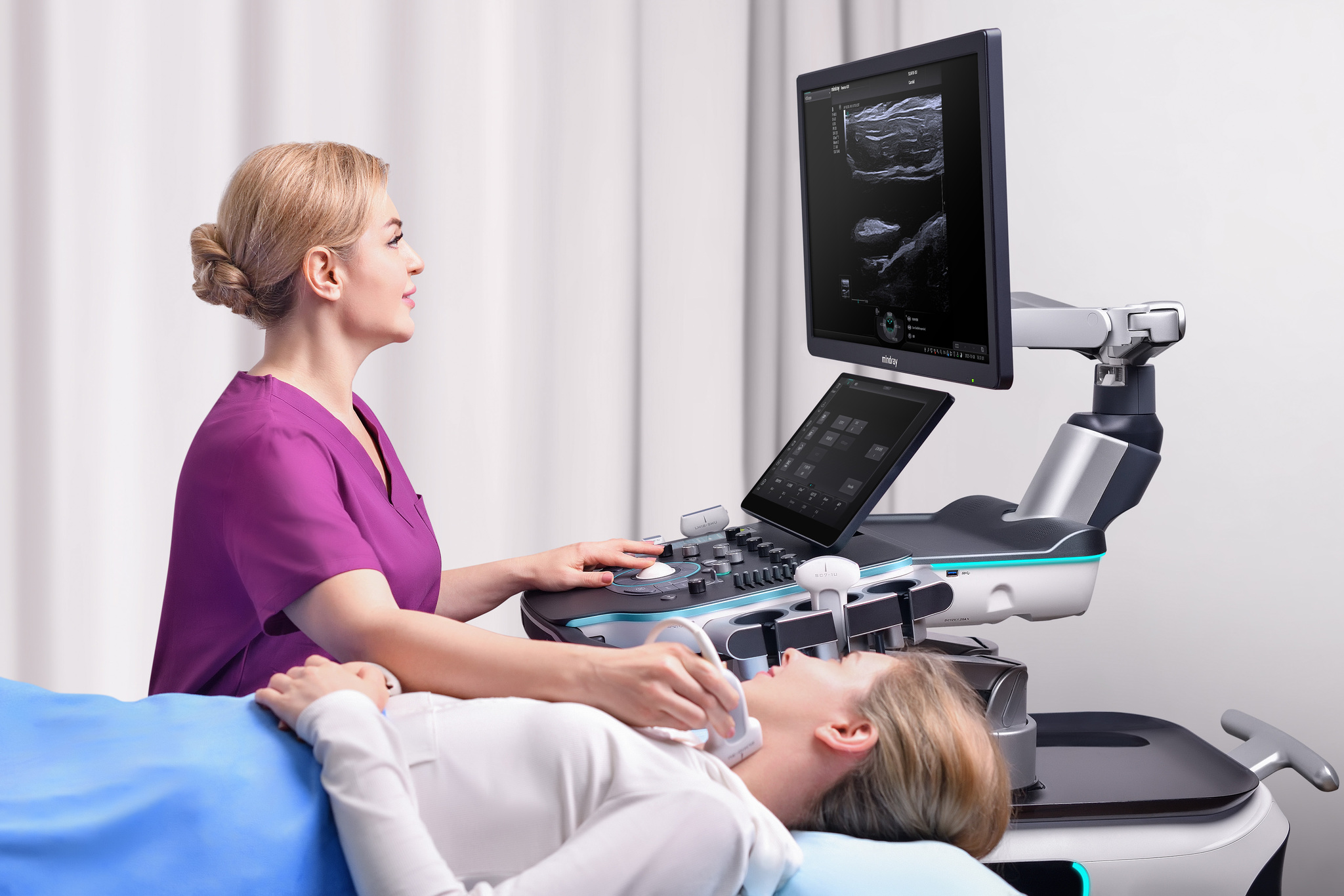
Shenzhen Mindray Bio-Medical Electronics Co., Ltd.The release of Resona A20 ultra-high-end ultrasound diagnostic system represents the highest level of medical imaging technology and design in China and marks that China's ultrasound system has officially entered the era of intelligent ultrasound. Thanks to the use of a series of innovative technologies in data, algorithm and computing power, Resona A20 has breakthrough innovative imaging technology and excellent ergonomic and aesthetic design. It is committed to restoring the real tissue structure, while helping to achieve the ultimate diagnosis of complicated diseases and exploring new directions of clinical research by experts.
Client / Manufacturer Design
Design

Shenzhen Mindray Bio-Medical Electronics Co., Ltd.
Shenzhen, CN
Shenzhen Mindray Bio-Medical Electronics Co., Ltd.
Shenzhen, CNJinzhou Zhao, Linghui Kuang, Ya Xu, Qi Zhang, Youxiang Wang, Rongfu Yang, Chen Cheng, Lang Lang, Xuanyi Li, Jiaxun LiaoDate of Launch
2023
Development Time
more than 24 Month
Target Regions
Asia, Australia / Oceania, Europe, North America, South America
Target Groups
Consumers / Users, "Medical Institutions"