












Stryker SurgiCount +
Medical device
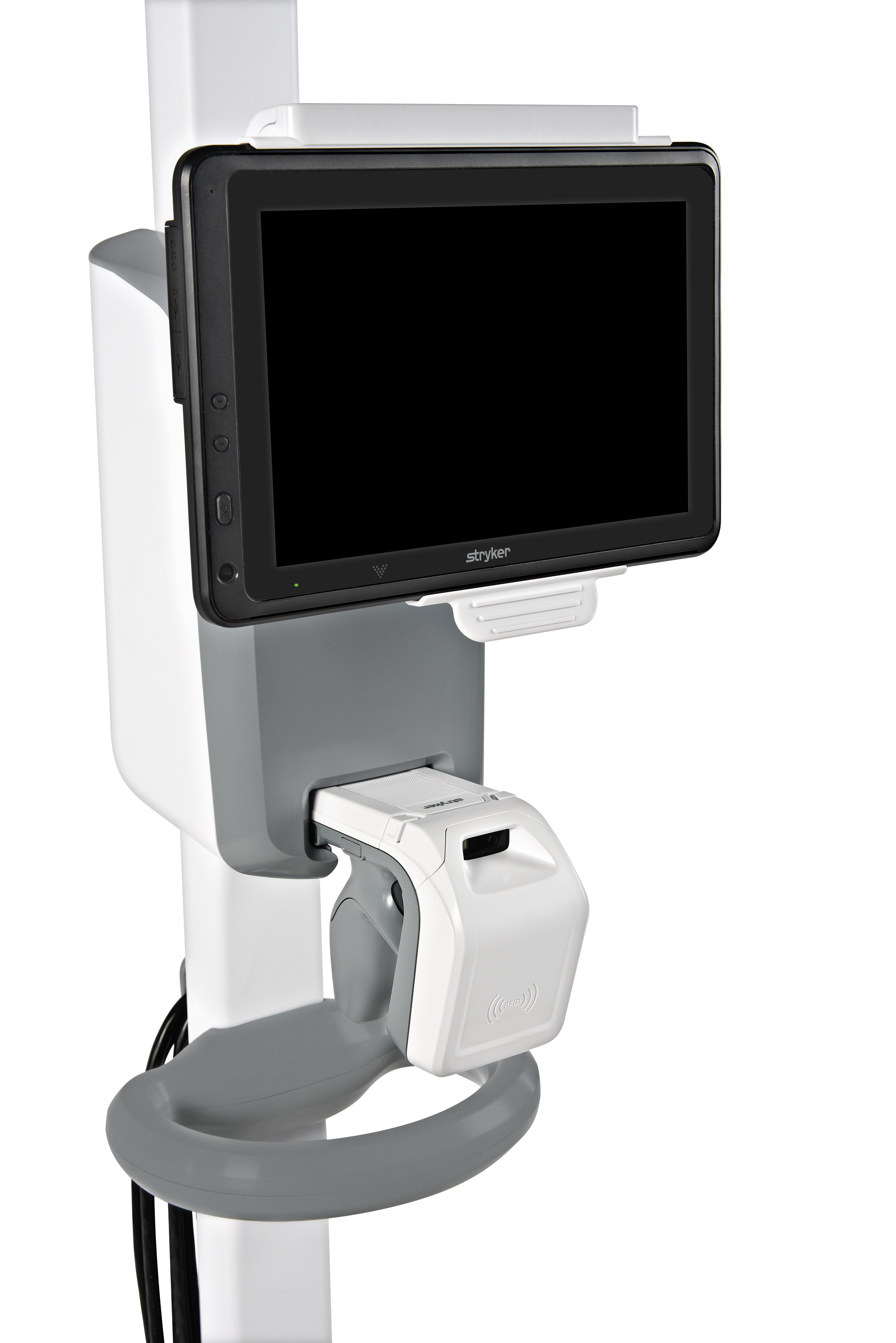
Stryker CorporationIn the United States, retained sponges are the No. 1 never event within surgical procedures, occurring over 4,000 times a year and costing over $600,000 per retained sponge in associated medical and legal expenses. SurgiCount+ is designed to improve workflow and prevent never events involving retained sponges. Each sponge has its own identifiable RFID that is scanned into and out of the surgical field. If a sponge is missing, the scanner can simply be removed from its holder and used as a tracker to find sponges within and outside of the patient anywhere within the operating room—essentially eliminating the risk of retained sponges.
Client / Manufacturer Design
Design

Stryker Corporation
Portage, USStryker Product Development Team DELVE Design
Portage, USDate of Launch
2021
Development Time
25 - 36 months
Target Regions
Asia, Europe, North America
Target Groups
Consumer / User